

Contains functions for working with reaction rate coefficients. The
calculateMicrocanonicalRateCoefficients() function is used to calculate
the forward and reverse microcanonical rate coefficients 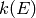 for each
path reaction. The fitInterpolationModel() function is used to fit an
interpolation model to a phenomenological rate coefficient k(T,P)() for
each net reaction.
for each
path reaction. The fitInterpolationModel() function is used to fit an
interpolation model to a phenomenological rate coefficient k(T,P)() for
each net reaction.
Calculate the microcanonical rate coefficient 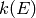 for the reaction
reaction at the energies Elist in J/mol. reacDensStates and
prodDensStates are the densities of states of the reactant and product
configurations for this reaction. If the reaction is irreversible, only the
reactant density of states is required; if the reaction is reversible, then
both are required. This function will try to use the best method that it
can based on the input data available:
for the reaction
reaction at the energies Elist in J/mol. reacDensStates and
prodDensStates are the densities of states of the reactant and product
configurations for this reaction. If the reaction is irreversible, only the
reactant density of states is required; if the reaction is reversible, then
both are required. This function will try to use the best method that it
can based on the input data available:
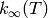 have been provided, then the inverse Laplace
transform method will be used.
have been provided, then the inverse Laplace
transform method will be used.The density of states for the product prodDensStates and the temperature of interest T in K can also be provided. For isomerization and association reactions prodDensStates is required; for dissociation reactions it is optional. The temperature is used if provided in the detailed balance expression to determine the reverse kinetics, and in certain cases in the inverse Laplace transform method.
Calculate the microcanonical rate coefficient for a reaction using RRKM theory, where transitionState is the transition state of the reaction, Elist is the array of energies in J/mol at which to evaluate the microcanonial rate, and densStates is the density of states of the reactant.
Calculate the microcanonical rate coefficient for a reaction using the inverse Laplace transform method, where kinetics is the high pressure limit rate coefficient, E0 is the ground-state energy of the transition state, Elist is the array of energies in J/mol at which to evaluate the microcanonial rate, and densStates is the density of states of the reactant. The temperature T in K is not required, and is only used when the temperature exponent of the Arrhenius expression is negative (for which the inverse transform is undefined).
For a set of phenomenological rate coefficients K computed at a grid of
temperatures Tlist in K and pressures Plist in Pa, fit a 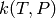 interpolation model, a tuple where the first item is a string describing
the type of model - either 'chebyshev' or 'pdeparrhenius' - and the
remaining elements contain parameters for that model. For Chebyshev
polynomials, the parameters are the number of terms to use in each of the
temperature and pressure dimensions. For pressure-dependent Arrhenius models
there are no additional parameters. Tmin, Tmax, Pmin, and Pmax
specify the temperature and pressure ranges in K and Pa, respectively,
over which the interpolation model is valid. If errorCheck is True,
a check will be performed to ensure that the interpolation model does not
deviate too much from the data; as this is not necessarily a fast process,
it is optional.
interpolation model, a tuple where the first item is a string describing
the type of model - either 'chebyshev' or 'pdeparrhenius' - and the
remaining elements contain parameters for that model. For Chebyshev
polynomials, the parameters are the number of terms to use in each of the
temperature and pressure dimensions. For pressure-dependent Arrhenius models
there are no additional parameters. Tmin, Tmax, Pmin, and Pmax
specify the temperature and pressure ranges in K and Pa, respectively,
over which the interpolation model is valid. If errorCheck is True,
a check will be performed to ensure that the interpolation model does not
deviate too much from the data; as this is not necessarily a fast process,
it is optional.